Some rockhounds like to collect rocks and minerals the size of small cars, or at least motorcycles. You know who you are (and so do the rest of us!). Others settle for samples that can be carried, a size that seems to decrease with each collecting season. But we also know of those who collect thumbnail specimens (less than 1”) and stick them into tiny boxes. There are even folks who like to collect specimens that require microscopes to be seen; these collectors are called micromounters. But I am going to tell you a bit about some “mineral specimens” that are even smaller. And I am even going to make up a new name for them. I am calling them micro-micro minerals.
When crystals grow they often do so imperfectly and they can trap material, often along the planes of growth. These can be solid material, rutilated quartz, star garnet, anthraxolite or other hydrocarbons, etc., or they can be the actual fluid that the crystal was growing from. Mineral collectors have a name for minerals where these internal fluids are visible. They call them enhydros and marvel at how the fluid or vapor bubble moves within the inclusion as the specimen is rotated. Geologists refer to them as fluid inclusions and study them for their scientific value.
There is often both a liquid and gas phase within an aqueous fluid inclusion. This is because the fluid was trapped at high temperature and pressure and upon cooling the liquid contracts and a vapor bubble forms. Geologists can heat the inclusion and watch the bubble decrease in size and eventually disappear. In most cases this tells them the minimum temperature at which the crystal grew. Other gases such as carbon dioxide and various hydrocarbon phases can form as the mineral and its liquid inclusions cool. But there can be more than liquid and vapor inside a fluid inclusion/enhydro.
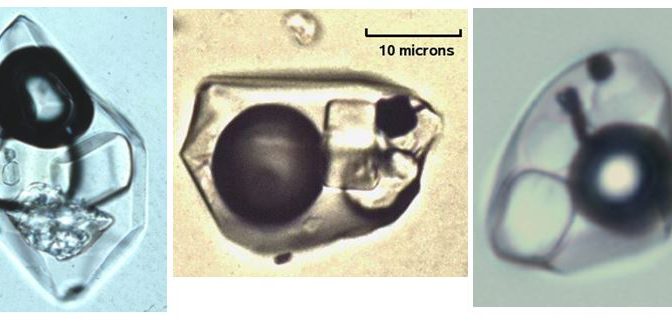
Hot fluids migrating within the earth’s crust or being released by a cooling magma are often saturated in various minerals. They are often saturated in salts (like halite-NaCl or sylvite-KCl). Upon cooling, salt crystals begin to precipitate within the inclusion. Tiny, micron-sized cubes of halite can grow. [A micron is 1/1000th of a millimeter, i.e. very small!] These micro-micro minerals can be viewed in thin polished sections under powerful microscopes and identified by their form and character or sometimes by electron microscopes. Geologists call these daughter minerals because they are younger than the inclusion within which they reside. They can be very useful indicators of the type of solution from which a mineral grew.

There are more exotic minerals found in some inclusions. Granitic magmas contain metals as dissolved elements, but these elements do not fit into the lattice structure of the primary minerals that crystallize from magmas (feldspars, quartz, pyroxenes, etc). As magmas cool to form igneous rocks like granite, metals are concentrated into a water phase that separates from the magma. These waters have very high salinity which permits the metals (copper, lead zinc, etc.) to be dissolved in solution as positively charged cations balanced by negatively charged chloride ions (Cl).
When these waters are released from the magma (the geologic term is exsolve), they often do so violently, and fracture the surrounding rocks. The vapors/waters move through these rocks and cool quickly while depositing minerals in the fractures to form mineralized veins. The fluids are silica-rich and quartz is the dominant mineral, but metal bearing sulfides are also deposited. The large porphyry copper deposits of the southwest United States are formed by this process with chalcopyrite being deposited in the fractures. Later the copper sulfides can be oxidized into the wondrous blues and greens of azurite, malachite, chrysocolla and turquoise we seek from places like Bisbee, Arizona or Chuquicamata, Chile. But that is another story.
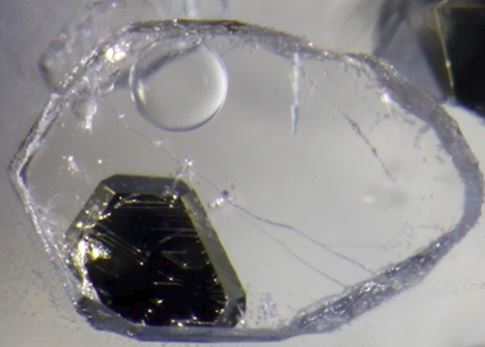
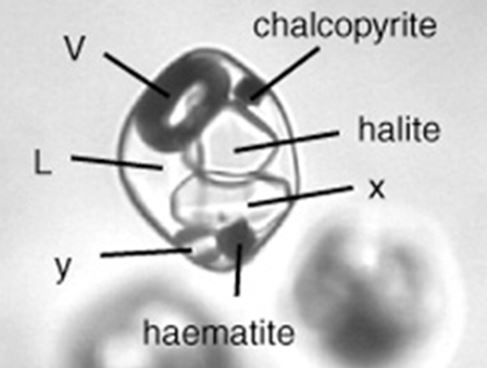
Fluid inclusions in quartz from hydrothermal ore deposits can be full of exotic daughter minerals. With original temperatures at the time of entrapment of several hundred degrees centigrade, these fluids often carry large quantities of salt and metal ions in solution. As they cooled, they lose their capacity to hold ions in solution. Minerals like chalcopyrite, hematite, and gypsum can form in fluid inclusions along with the more common salts, halite and sylvite.

Inclusions containing micro-micro/ daughter minerals are neat to view in the microscope, but as you look at the pictures in this note, it is interesting to think for a moment about the geologic history that is stored in those little drops of fluid and the solids that formed within it after it was trapped. If the tiny drop of fluid was trapped when the crystal grew (geologist’s refer to these as primary fluid inclusions) then it has been chemically isolated for hundreds of millions of years or more depending on the age of the mineralization.
The mineral hosting a fluid inclusion may have formed at great depth and only have been recently exposed after eons of uplift and erosion. Inclusions in quartz pebbles within a conglomerate will reflect the origin of quartz that predates the formation of the rock within which it now resides. But inclusions in a wide variety of rocks permit the history of the mineral/rock to be unraveled if analyzed properly.
Gemologists observe the fluid inclusions in sapphires and other gems to learn if they have been heat treated to enhance their color (Koivula, 1986). The inclusions can not survive the heating required to alter the color of a sapphire. If the inclusions are intact and still contain CO2 then the gem has not been altered.
Is it any wonder that geologists seek out, and literally salivate, over the opportunity to study the content of fluid inclusions from their favorite ore deposit or geologic project?
References:
Koivula, J. I., 1986, Carbon Dioxide fluid Inclusions as Proof of Natural-Colored Courundum, Gems and Gemology, Fall, 1986, p. 152-155.
Mei, W., et. al., 2015, Ore genesis and hydrothermal evolution of the Huanggang skarn iron-tin polymetallic deposit, southern Great Xing’an Range: Evidence from fluid inclusions and isotope analyses, Ore Deposit Reviews, V. 64, P. 239-252.
Renfro, Koivula, J. I., and Skalwold, E. A., 2016, A Fantastic Display of Phase Changes in a Sapphire’s Fluid Incluson, Gems and Gemology, V. 52, no. 1
Ulrich, T., Gunther, D., and Heinrich, D.A., 1999, Gold concentrations of magmatic brines and the metal budget of porphyry copper deposits, Nature, v. 399, p. 676-679.
Walter, M., 2014, Collector’s Guide to Herkimer Diamonds, Schiffer Earth Science Monograph Volume #18, 94 p.