On July 4th, we celebrated our nation’s independence with fireworks. This year many public displays were canceled, but if your neighborhood was like mine, there were still plenty of pyrotechnics decorating the evening sky. The wonderful colors are all part of simple elemental chemistry and most owe their origin to minerals and chemical compounds (metal salts) produced from those minerals.
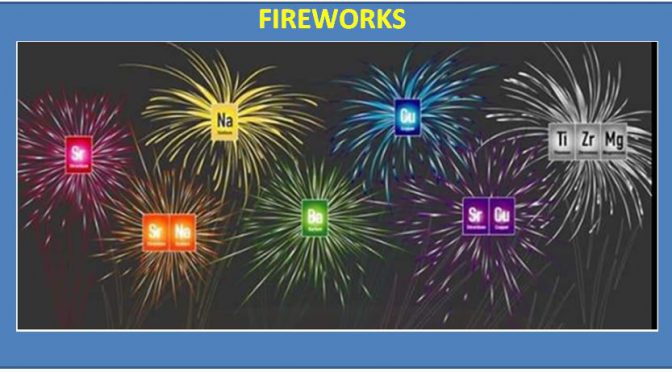
There are exceptions, but most fireworks rely on specific elements to produce the colorful displays. Color variations can be created when the elemental compounds are combined.
- Red fireworks are produced from strontium carbonate
- Yellow fireworks generally require sodium nitrate
- Green colors result from fireworks containing barium chloride
- Blue fireworks are produced by copper chloride
- White or silver colors require titanium or magnesium salts
- Orange colors can be produced by calcium chloride (or by mixing strontium and sodium compounds)
- Purple fireworks combine copper with strontium compounds
All of these chemicals used in fireworks are compounds called “salts”. A salt is any compound that contains both a metal and non-metal atom. When certain salts are burned they display intense color that is derived from the metal in the compound; these colors are defined as the flame color for the metal. Sulfur and charcoal along with potassium nitrate are added as the “lift charge” and to help the fireworks burn. Nitrates, chlorates, and perchlorates are included in the mixture to provide oxygen for combustion Dextrin is added to hold the mixture together. Fireworks are clearly a complicated mixture of chemicals, but it is the metal content of the salt that dictates the color.
Many of the minerals from which these metals are recovered can be collected locally or otherwise acquired. There is strontianite at Mt. Pleasant Mills in PA, and barium is often recovered from the sulfate mineral barite. Is it hard to imagine that brilliant blue comes from copper: just think of azurite, chrysocolla or even turquoise? Most titanium is recovered from rutile or ilmenite, but there is titanium in titanite also.
Most fireworks store all their chemicals in a pea- to peach-sized pellet. The lift charge is ignited to lift the firework into the air and a timed fuse is lit simultaneously. The final explosion of color occurs several seconds later when the burning fuse reaches and ignites the core mixture of compounds, hopefully when the charge is high above the ground, out of harm’s way, and visible to all below.
References:
Conners, D, and Anderson, P.S., 2020, How do Fireworks Get Their Color, Human World webpage
www.usgs.gov/minerals, 2020, What minerals produce the colors in fireworks?