Published in May 2018 Wayne County Gem and Mineral Newsletter
Contrary to what you may have thought in high school, the Periodic Table of the Elements was not created to torture high school chemistry students. The idea was simple at first. Aristotle considered a four element table way back in 330 BCE. He identified earth, air, fire and water as the four elemental building blocks. But others since have not been satisfied with his simple approach, and the list grew and with that came complexity.
By the 1700s some 30 elements had been isolated and described and that grew to over 60 by the middle of the 19th century. And it was in the middle of the 19th century that several chemists and inventors working independently began to recognize patterns in those elements and began to organize them into lists and tables. A Russian chemist named Dmitri Mendeleev had the foresight to recognize that the known elements should be organized on their atomic weight and he also set the elements into a two-dimensional grid based on common properties. But it was his observations that the chart needed gaps for elements that had yet to be discovered that set him apart from others. For this insight, Mendeleev is credited as the “father of the Periodic Table”.
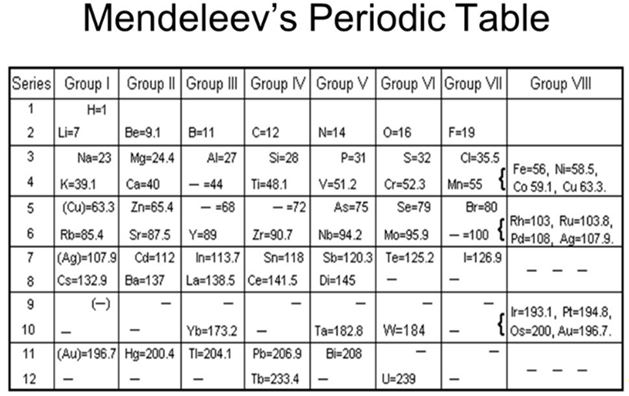

Now it is 2019, and the 150th anniversary of the Periodic Table. To celebrate the Sesquicentennial of Mendeleev’s remarkable achievement the United Nations has proclaimed 2019 as the International Year of the Periodic Table of the Elements. (IYPT 2019). In their announcement, the UN identifies this achievement as “one of the most significant achievements in science”.
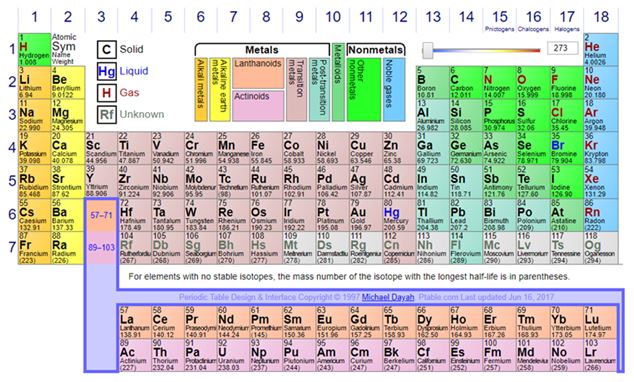

Mineral collectors don’t collect minerals containing oganesson, but we do collect minerals with a significant number of elements. I wonder how many different elements are in the minerals in your collection. Some are simple native elements, perhaps you have some gold (Au). Most of us have some native copper (Cu). Some of us even hope to collect some in Michigan this summer. And of course, some elements come in two different forms. Graphite is carbon (C) formed in the earth’s crust at relatively low temperature and pressure and diamonds are a form of carbon formed within the earth’s mantle at very high pressure.
Do you have any tourmaline? There might be ten different elements hiding in your tourmaline. Take a look at your collection. How many elements can you identify among your mineral specimens? Do you have any tungsten (W) minerals? Do you have any with phosphorus (P) or vanadium (V)? Which ones are they? It would be interesting to organize your minerals by their chemistry and then perhaps look for new ones to fill holes, much as Mendeleev had to do. And what better year to do that than during the Sesquicentennial celebration of the Periodic Table!
Did you know that the manufacture of your cell phone requires over 30 different elements, many of which were not even known when Mendeleev created his initial Periodic Table? From the lightest metals [lithium (Li) and beryllium (Be)] to heavier rare-earth elements like lanthanum (La) and neodymium (Nd) the specific functionality in many of our common electronic devices is enabled by the varying properties of these elements. Currently, nine of the metals required for cell phone technology are regarded as “critical metals” by the US Department of Defense. These are metals we cannot live without. Many currently have singular global sources. Another 13 of the elements are listed as specialty metals. These are used in the high-tech applications but are not well known in the mineral world: elements like indium (In) and gallium (Ga), two that Mendeleev predicted, but could not identify or Tantalum (Ta) which can be collected in some pegmatite minerals. Do you have any minerals with these elements in your collection?

References:
Development of the Periodic Table, Royal Society of Chemistry Webpage
International Year of the Periodic Table (IYPT 2016) webpage
Turel, I., 2019, 150th anniversary of the periodic table of chemical elements, periodic table, Mendeelev and philately, webpage
Various Wikipedia webpages